Abstract
Introduction: Disease cytogenetics have been shown to predict chemo-responsiveness and likelihood of relapse in children and young adults with B-cell acute lymphoblastic leukemia (B-ALL). NCCN defines ETV6/RUNX1 and Hyperdiploid ALL as low risk (LR) cytogenetics, and high-risk (HR) cytogenetics include Philadelphia Chromosome positive (Ph+) and Ph-like, KMT2A-rearrangement (KMT2A-r), Hypodiploid, TCF3-HLF, and iAMP-21. HR cytogenetics are thought to render leukemic cells resistant to chemotherapy limiting the efficacy of upfront and salvage treatment. Unlike chemotherapy, chimeric antigen receptor (CAR) T-cell therapy leverages a cytotoxic CD8+ T-cell response for disease clearance, and existing cytogenetic risk stratification may not be predictive of CAR response and durability of remissions. We aimed to study the landscape of standard cytogenetic risk stratification in patients treated with commercial CD19-CAR T cell therapy (tisagenlecleucel) to evaluate associated post-CAR outcomes.
Methods: Retrospective data was abstracted from the Pediatric Real-World CAR Consortium (PRWCC) database. Data across 185 infused patients from 15 oncology sites were collected including; baseline demographics, cytogenetic classification, prior therapy, baseline disease burden (high burden; ≥5% bone marrow blasts, low burden; <5%) and post-CAR response, relapse and survival outcomes.
Results: 141/185 CAR-treated patients in the PRWCC database had available cytogenetic data for analysis. The cohort included 25 patients with low-risk cytogenetics, 68 with high-risk cytogenetics and 48 with neutral cytogenetics per NCCN guidelines.
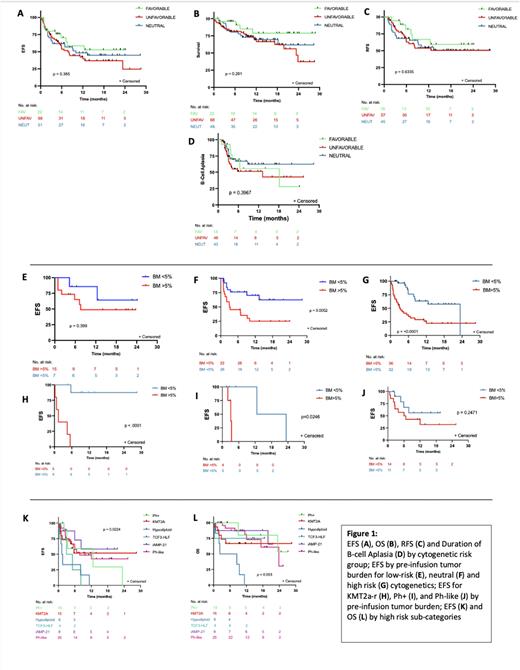
The day 28 complete remission (CR) rate for all patients was 83.7% (118/141) with no significant difference (p=0.28) between LR (84%, 21/24), HR (82.4%, 56/68) or neutral cytogenetics (89.6%, 43/48). Additionally, no difference was found between cytogenetic risk groups in regards to overall survival (p=0.22), event free survival (EFS) (p=0.38), relapse free survival (p=0.91) or duration of B-cell aplasia (p=0.40) (Figure 1). Both overall relapse (p=0.19) and CD19-negative relapse rates (p=0.77) were not statistically different between risk groups, and there was no difference in toxicity for both cytokine release syndrome (p=0.75) or neurotoxicity (p=0.13).
Compared to patients with LR or neutral cytogenetics, the HR patient group proceeded to CAR sooner from time of diagnosis (16.5 mo vs 37 mo, p= 0.03) and had a lower median CAR dose (p=0.014). When controlling for the KMT2A-r cohort, patients with HR cytogenetics also tended to be older at time of diagnosis (p<0.001) and CAR infusion (p=0.001). No difference was noted between the cytogenetic risk categories and several factors known to modulate CD19 CAR outcomes, including disease burden, prior blinatumomab exposure/response, primary refractory disease, and median lines of therapy pre-CAR including prior transplant exposure.
Notably, distinct cytogenetic groups were variably impacted by disease burden. Patients with neutral or HR cytogenetics had significantly inferior median EFS when baseline disease burden was high (neutral; 3.1 months (p=0.005), HR; 3.1 months (p=0.001)) versus low (neutral; not reached, HR; 23.4 months). Low burden disease did not result in significantly different outcomes for patients with low risk cytogenetics. Amongst high risk cytogenetics subgroups, the impact of disease burden was also not universal. Patients with Ph+ and KMT2A-r with lower disease burden had superior EFS, but low burden disease did not result in superior EFS for Ph-like disease (Figure 1). Two HR sub-categories with particularly poor outcomes were Hypodiploid (n=6) and TCF3-HLF (n=4) ALL. Patients with Hypodiploid ALL had a median EFS and OS post-CAR of only 1.4 and 5.6 months, respectively, and there were no long-term remissions post-CAR for patients with TCF3-HLF who had a median EFS of 4 months.
Conclusions: Traditional cytogenetic risk categories are not predictive of response to CD19 CAR therapy, as patients with historically high-risk disease classification have comparable outcomes to patients with low-risk or neutral cytogenetics. Distinct CAR-specific predictors such as high burden disease supplant established risk criteria but are also not uniform amongst cytogenetic sub-categories, thereby supporting the need to develop future CAR-specific risk-classification systems.
Disclosures
Stefanski:Novartis: Other: Ad Board, Speakers Bureau. Verneris:Novartis: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Consultancy, Current equity holder in private company; Up-To-Date: Consultancy; Bmogen: Consultancy, Current equity holder in private company. Myers:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eliana Trial: Consultancy, Membership on an entity's Board of Directors or advisory committees. Qayed:Vertex: Honoraria; Novartis: Honoraria. Hermiston:Sobi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Satwani:Mesoblast: Honoraria; Takeda: Honoraria. Curran:Novartis: Consultancy, Research Funding. Mackall:Syncopation: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Ensoma: Divested equity in a private or publicly-traded company in the past 24 months; Mammoth: Divested equity in a private or publicly-traded company in the past 24 months; Link: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; BMS: Consultancy; Immatics: Consultancy; Medimmune Tech: Consultancy; Nektar: Consultancy; Apricity: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Lyell Pharmaceuticals: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; GSK: Consultancy. Laetsch:Bayer: Consultancy, Research Funding; Cellectis: Consultancy; Pfizer: Research Funding; Novartis: Consultancy, Research Funding; Advanced Microbubbles: Current holder of stock options in a privately-held company; AI Therapeutics: Consultancy; Deciphera: Consultancy; GentiBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Jumo Health: Consultancy; Massive Bio: Consultancy; Menarini: Consultancy; Pyramid Biosciences: Consultancy; y-mAbs Therapeutics: Consultancy. Schultz:Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal